Welcome to part 8 of the ODX Stress Biomarkers Series. This post focuses on some of the individual biomarkers associated with the stress response. It is important to monitor these biomarkers if prolonged stress is suspected, as these changes can eventually become detrimental including hyperglycemia, hyperlipidemia, elevated aldosterone, and increased or decreased cortisol.
The ODX Stress Biomarkers Series
- Stress Part 1: A Quick Overview
- Stress Part 2: How Do We Get Stressed Out?
- Stress Part 3: The Physiology of Stress
- Stress Part 4: Hormonal Control of the Stress Response
- Stress Part 5: Identifying Stress
- Stress Part 6: Disorders Associated with Stress
- Stress Part 7: Biomarkers of Stress Overview
- Stress Part 8: Biomarkers of Stress, Individual Markers
- Stress Part 9: Addressing Stress: Can We Treat It or Beat It?
- Stress Part 10: Nutrition and Stress
- Stress Part 11: Optimal Takeaways
Cortisol
The glucocorticoid cortisol at first glance is a beneficial compound that decreases inflammation and mobilizes stored energy reserves for immediate use. Cortisol suppresses insulin, delays wound healing, and suppresses the immune inflammatory response in order to focus resources on the task at hand. However, prolonged cortisol stimulation will have detrimental catabolic and immune-suppressive effects.[i]
Cortisol production is regulated by the HPA axis. Corticotropin-releasing hormone (CRH) from the hypothalamus stimulates the anterior pituitary to produce adrenocorticotropic hormone (ACTH). It is ACTH that then stimulates the adrenal gland to produce and release cortisol. The subsequent increase in serum cortisol feeds back and reduces production of CRH and ACTH. Without this feedback, excess ACTH will signal the adrenal gland to continue to make cortisol. However, elevated ACTH will not result in increased cortisol production in adrenal insufficiency or hypofunction, and cortisol levels will remain insufficient. Failure of the adrenal gland to produce cortisol may be caused by damage, autoimmune disorder, congenital disease, or prolonged use of steroids which suppress adrenal function. Pathological insufficiency of cortisol is known as Addison’s disease while excess production of cortisol manifests as Cushing’s syndrome.[ii]
Cortisol normally increases in the early morning and then declines during the day and into nighttime. However, persistent elevations in cortisol, as seen with acute stress, can have detrimental effects throughout the body.[iii]
Elevated cortisol can promote beta cell dysfunction and impair glucose regulation. One cross-sectional population-based study of 1,071 Japanese participants found that a fasting morning cortisol above 11 ug/dL was significantly associated with reduced insulin secretion and beta cell function.[iv] Further evaluation should be completed to fully assess glucose regulation parameters if morning cortisol continues to increase.[v]
Stress is initially characterized by elevated cortisol and decreased DHEA (after a short-term initial increase), demonstrating a shift into stress hormone production. If prolonged, chronic stress can lead to adrenal exhaustion with low cortisol and low DHEA. Chronically elevated cortisol can also have adverse effects on the brain and contribute to anxiety and depression as cortisol shunts tryptophan away from the production of serotonin and melatonin.[vi]
The ratio of cortisol to DHEA or DHEA-S (the more abundant sulfated form of DHEA) may help predict tolerance to stress. A higher ratio of cortisol to DHEA was associated with increased arousal, anxiety, fear, and startle response in a clinical study of 30 volunteers. A lower ratio is believed to buffer the negative effects of acute stress.[vii] Interestingly, estradiol may temper the cortisol response to stress by increasing corticosteroid-binding globulin, therefore decreasing free cortisol.[viii]
DHEA, DHEA-S
Dehydroepiandrosterone (DHEA) is an anabolic steroid produced by the adrenal glands. The most abundant form in circulation is DHEA-S, the stable sulfated form. DHEA is a precursor to testosterone but also has restorative effects that support immunity, wellbeing, mood, and behavior. A meta-analysis of 14 studies indicates that DHEA increases with acute stress in response to adrenocorticotropic hormone. This protective response helps buffer the negative effects of cortisol and may support mood following acute mental stress. DHEA levels rise within the first hour, peak, and then decline. Elevations were more pronounced in younger individuals, obese individuals, and females.[ix]
In stress, DHEA is considered a biomarker of HPA axis activity along with CRH, ACTH, and cortisol. DHEA is able to buffer the effects of cortisol, especially persistently higher levels of cortisol that are associated with impaired cognitive function, anxiety, depression, and coronary heart disease. Not surprisingly, insufficient DHEA may exacerbate the negative effects of excess cortisol.[x]
Chronic or prolonged stress will lead to the downregulation of DHEA production, followed by downregulation of cortisol production, a pattern characteristic of adrenal hypofunction or exhaustion. The resulting increased cortisol to DHEA ratio has been reported in those exposed to long-term stress or experiencing clinical burnout. Increased cortisol:DHEA ratio has also been reported in association with workdays, increased stressful events in the year prior, overtraining, and caring for Alzheimer’s patients (Kamin 2017).[xi]
Aldosterone
Although not commonly thought of as a stress hormone, the adrenal mineralocorticoid aldosterone increases in response to stress and HPA activation and is further influenced by the sympathetic-adrenomedullary system. Research suggests that elevated aldosterone may be associated with increased risk of metabolic dysfunction and cardiovascular risk via a number of different actions as it: [xii]
- Increases water and sodium retention
- Facilitates potassium excretion
- Increases risk of hypertension
- Increases pro-inflammatory mediators
- Decreases adiponectin and insulin sensitivity
- Activates the mineralocorticoid receptor (MR)
- Levels increase with sodium intake
- Levels increase with stress
- Increased aldosterone may contribute to mood disorders including anxiety and depression
The link between aldosterone and essential hypertension was investigated in a study of 113 hypertensive subjects who did not have primary aldosteronism. In those with an exaggerated response to stress/ACTH stimulation, blood pressure was normalized with the use of mineralocorticoid receptor antagonists.[xiii]
Aldosterone’s effect on the immune system may be a link to its cardiovascular effects as well. Excess aldosterone can activate immune cells and promote a Th17-based immune response that contributes to hypertension, vascular injury, and cardiovascular fibrosis.[xiv]
Sodium and Potassium
Stress-induced increases in aldosterone will affect sodium-potassium balance by causing sodium and water retention and potassium excretion. An increase in plasma sodium:potassium ratio is associated with significantly increased risk of hypertension as demonstrated in a community study of 549 volunteers. Assessing the ratio of sodium to potassium was more useful than assessing either electrolyte alone. The study also noted that hypertensive subjects had significantly increased levels of hs-CRP, ferritin, and malondialdehyde, and significantly decreased glutathione, SOD, NO, and catalase.[xv]
An increased serum sodium:potassium ratio, especially above 34, suggests a potassium depletion. A ratio below 28 suggests sodium depletion as well as insufficiency of magnesium and vitamin E. [xvi] An elevated sodium:potassium ratio may be indicative of acute stress while a decrease in sodium:potassium ratio may occur with chronic stress and adrenal insufficiency.
Interestingly, a low-salt diet can activate the sympathetic nervous system and stimulate production of aldosterone. A study of 152 healthy subjects found that a low-salt diet was associated with significantly increased serum aldosterone with a mean of 21 ng/dL on the low-salt diet versus 3.4 ng/dL on the high-salt diet. Researchers associated a low-salt diet with insulin resistance in this cohort.[xvii]
Glucose
Stress triggers a short-term elevation in blood glucose that can be used for energy.
- The glucocorticoid cortisol promotes the conversion of protein/amino acids to glucose through the process of gluconeogenesis.
- Pancreatic glucagon also increases serum glucose by stimulating gluconeogenesis and glycogen breakdown in the liver.[xviii]
- Both epinephrine and norepinephrine will promote an increase in blood glucose.[xix]
- Even moderate elevation in fasting blood glucose at 95-99 mg/dL (5.27-5.49 mmol/L) can increase cardiovascular risk significantly compared to lower levels below 80 mg/dL (4.44 mmol/L).[xx]
Blood glucose can increase in situations that may not traditionally be thought of as stress. These include myocardial infarction, stroke, anesthesia, burns, and strenuous exercise.[xxi] Even exposure to stress during pregnancy can increase the chance of blood glucose dysregulation in the child.[xxii]
Lipids
Stress is also associated with alterations in serum lipids. Review of the data from 9,752 individuals taking part in the Isfahan Healthy Heart Program found that elevated psychological stress was associated with significant elevations in total and LDL cholesterol and decreases in HDL-C. Researchers note that previous studies had also demonstrated an increase in triglyceride levels associated with stress.[xxiii]
In one historical cohort study of ~5,000 subjects, researchers note that both physical and psychological stress can alter serum lipids, especially with advancing age and obesity. Triglycerides and LDL were highest, and HDL lowest, in subjects with heavy physical activity/stress. This group had the greatest number of subjects with triglycerides greater than 200 mg/dL (2.26 mmol/L), LDL greater than 130 mg/dL (3.37 mmol/L), and HDL below 45 mg/dL (1.17 mmol/L). Total cholesterol above 200 mg/dl (5.18 mmol/L) was most prevalent in subjects with both psychological stress and physical work/stress.[xxiv]
Another study of 208 undergraduate students found that the examination period, a source of psychological stress, was associated with significantly increased cortisol, epinephrine, total cholesterol, HDL-C, and LDL-C.[xxv]
Oxidative Stress
The stress response involves interactions between immune cytokines and the HPA axis which result in biochemical and physiological changes. Ultimately the cardiovascular, nervous, renal, hepato-biliary, and digestive systems become involved.
Generation of oxidative stress and corresponding antioxidant and inflammatory activity is also a hallmark of stress. An overwhelming imbalance that favors oxidative stress will be detrimental to DNA and RNA and eventually cause cell and organ dysfunction. Red blood cells are particularly vulnerable to oxidative stress and can be assessed with a complete blood count (CBC).
Oxidative stress can be countered by antioxidants and endogenous antioxidant enzymes including catalase, glutathione peroxidase, glutathione reductase, and superoxide dismutase. Of course, prevention is preferable, and therefore causes of oxidative stress should be minimized. These causes include:[xxvi]
- Nutrient depletions, poor diet
- Pathological microbes
- Pollutants
- Radiation, ionizing and nonionizing
- Toxic gasses, ozone, oxidizing disinfectants
- Toxins, biological and chemica
Schematic representation of various endogenous and exogenous factors that act as stressors and lead to the generation of ROS and oxidative stress/modification.
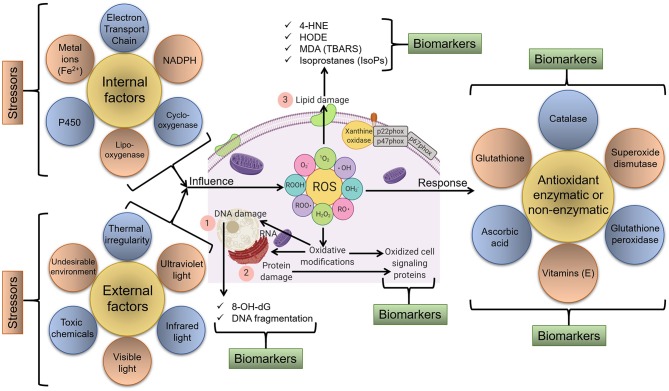
In response, various molecular and cellular redox-sensitive processes start that can be tracked as biomarkers of oxidative stress. For instance, major biomarkers include (1) markers of DNA/RNA damage/oxidation, (2) markers of protein damage/oxidation, and (3) markers of lipid damage via the oxidation of membrane components and available lipids, etc.
Source: Dhama, Kuldeep et al. “Biomarkers in Stress Related Diseases/Disorders: Diagnostic, Prognostic, and Therapeutic Values.” Frontiers in molecular biosciences vol. 6 91. 18 Oct. 2019, doi:10.3389/fmolb.2019.00091 This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY).
Salivary measurement of stress biomarkers
Stress markers may also be measured and monitored in saliva as concentrations often mirror circulating levels.[xxvii] Cortisol and DHEA can be easily measured in blood or saliva. Another marker, salivary alpha-amylase (SAA), appears to parallel stress-induced increases in norepinephrine and catecholamine activity. Researchers suggest that SAA may be a surrogate marker for activation of the sympathetic-adreno-medullary axis.[xxviii]
Salivary measurement of cortisol, DHEA-S, and SAA in a study of young healthy males demonstrates a normal pattern of activity including: [xxix]
- Cortisol increases significantly upon awakening and then decreases 45 minutes later, reaching nadir in the evening.
- DHEA-S starts relatively high in the morning, decreases for the first 60 minutes after awakening, and then steadily decreases until nadir in the evening.
- Alpha-amylase decreases immediately upon awakening, returns to baseline 30-60 minutes after awakening, and then increases during the day, reaching peak in the evening.
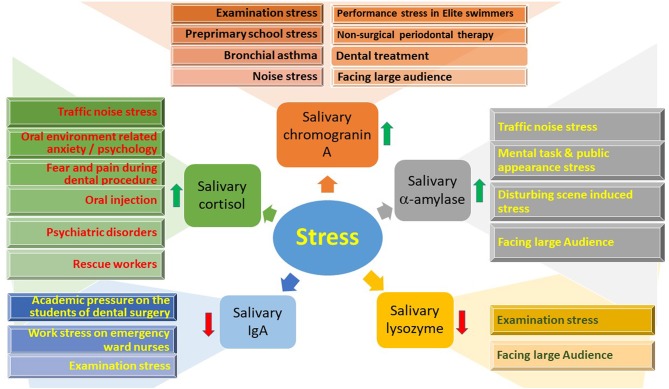
Various stresses affecting the salivary stress biomarkers: cortisol, chromogragranin A and a-amylase are increased whilst salivary IgA and lysozyme are decreased with an increase in level of stress.
Source: Dhama, Kuldeep et al. “Biomarkers in Stress Related Diseases/Disorders: Diagnostic, Prognostic, and Therapeutic Values.” Frontiers in molecular biosciences vol. 6 91. 18 Oct. 2019, doi:10.3389/fmolb.2019.00091 This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY).
However, measurement of inflammatory cytokines in saliva may not accurately mirror circulating levels, especially if oral infection or inflammation is present. Cytokines are larger molecules and are less stable than cortisol. Salivary CRP may have some correlation with blood levels as it is not produced locally in the mouth. However, research into the reliability of salivary CRP as a marker of stress is currently limited.[xxx]
Monitoring the biomarkers of stress can be an important part of stress management, especially in individuals exposed to an exceptionally high level of stress, or in individuals with a very low tolerance to stress.
References
[i] Chu, Brianna, et al. “Physiology, Stress Reaction.” StatPearls, StatPearls Publishing, 8 June 2021.
[ii] Pagana, Kathleen Deska; Pagana, Timothy J.; Pagana, Theresa N. Mosby's Diagnostic and Laboratory Test Reference. Elsevier Health Sciences. 2019.
[iii] Noushad, Shamoon et al. “Physiological biomarkers of chronic stress: A systematic review.” International journal of health sciences vol. 15,5 (2021): 46-59.
[iv] Kamba, Aya et al. “Association between Higher Serum Cortisol Levels and Decreased Insulin Secretion in a General Population.” PloS one vol. 11,11 e0166077. 18 Nov. 2016, doi:10.1371/journal.pone.0166077
[v] Kamba, Aya et al. “Association between Higher Serum Cortisol Levels and Decreased Insulin Secretion in a General Population.” PloS one vol. 11,11 e0166077. 18 Nov. 2016, doi:10.1371/journal.pone.0166077
[vi] Murray, Michael T., and Joseph Pizzorno. The encyclopedia of natural medicine third edition. Simon and Schuster, 2012.
[vii] Grillon, Christian et al. “Cortisol and DHEA-S are associated with startle potentiation during aversive conditioning in humans.” Psychopharmacology vol. 186,3 (2006): 434-41. doi:10.1007/s00213-005-0124-2
[viii] Hariri, Lana. and Anis Rehman. “Estradiol.” StatPearls, StatPearls Publishing, 13 February 2021. This book is distributed under the terms of the Creative Commons Attribution 4.0 International License (),
[ix] Dutheil, Frédéric et al. “DHEA as a Biomarker of Stress: A Systematic Review and Meta-Analysis.” Frontiers in psychiatry vol. 12 688367. 6 Jul. 2021, doi:10.3389/fpsyt.2021.688367
[x] Piazza, Jennifer R et al. “Frontiers in the use of biomarkers of health in research on stress and aging.” The journals of gerontology. Series B, Psychological sciences and social sciences vol. 65,5 (2010): 513-25. doi:10.1093/geronb/gbq049
[xi] Kamin, Hayley S, and Darlene A Kertes. “Cortisol and DHEA in development and psychopathology.” Hormones and behavior vol. 89 (2017): 69-85. doi:10.1016/j.yhbeh.2016.11.018
[xii] Kubzansky, Laura D, and Gail K Adler. “Aldosterone: a forgotten mediator of the relationship between psychological stress and heart disease.” Neuroscience and biobehavioral reviews vol. 34,1 (2010): 80-6. doi:10.1016/j.neubiorev.2009.07.005
[xiii] Markou, Athina et al. “Stress-induced Aldosterone Hyper-Secretion in a Substantial Subset of Patients With Essential Hypertension.” The Journal of clinical endocrinology and metabolism vol. 100,8 (2015): 2857-64. doi:10.1210/jc.2015-1268
[xiv] Ferreira, Nathanne S et al. “Aldosterone, Inflammation, Immune System, and Hypertension.” American journal of hypertension vol. 34,1 (2021): 15-27. doi:10.1093/ajh/hpaa137
[xv] Ekun, Oloruntoba A et al. “Assessment of Plasma Sodium to Potassium Ratio, Renal Function, Markers of Oxidative Stress, Inflammation, and Endothelial Dysfunction in Nigerian Hypertensive Patients.” International journal of hypertension vol. 2020 6365947. 7 Dec. 2020, doi:10.1155/2020/6365947
[xvi] Wardle, Jon, and Jerome Sarris. Clinical naturopathy: an evidence-based guide to practice. Elsevier Health Sciences, 2019. 3rd edition.
[xvii] Garg, Rajesh et al. “Low-salt diet increases insulin resistance in healthy subjects.” Metabolism: clinical and experimental vol. 60,7 (2011): 965-8. doi:10.1016/j.metabol.2010.09.005
[xviii] Singh, K. "Nutrient and stress management." J Nutr Food Sci 6.4 (2016): 528.
[xix] Piazza, Jennifer R et al. “Frontiers in the use of biomarkers of health in research on stress and aging.” The journals of gerontology. Series B, Psychological sciences and social sciences vol. 65,5 (2010): 513-25. doi:10.1093/geronb/gbq049
[xx] Shaye, Kivity et al. “Fasting glucose levels within the high normal range predict cardiovascular outcome.” American heart journal vol. 164,1 (2012): 111-6. doi:10.1016/j.ahj.2012.03.023
[xxi] Pagana, Kathleen Deska; Pagana, Timothy J.; Pagana, Theresa N. Mosby's Diagnostic and Laboratory Test Reference. Elsevier Health Sciences. 2019.
[xxii] Entringer, Sonja. “Impact of stress and stress physiology during pregnancy on child metabolic function and obesity risk.” Current opinion in clinical nutrition and metabolic care vol. 16,3 (2013): 320-7. doi:10.1097/MCO.0b013e32835e8d80
[xxiii] Shahnam, Maryam et al. “The correlation between lipid profile and stress levels in central iran: isfahan healthy heart program.” ARYA atherosclerosis vol. 6,3 (2010): 102-6.
[xxiv] Assadi, Seyedeh Negar. “What are the effects of psychological stress and physical work on blood lipid profiles?.” Medicine vol. 96,18 (2017): e6816. doi:10.1097/MD.0000000000006816
[xxv] Maduka, Ignatius C et al. “The relationship between serum cortisol, adrenaline, blood glucose and lipid profile of undergraduate students under examination stress.” African health sciences vol. 15,1 (2015): 131-6. doi:10.4314/ahs.v15i1.18
[xxvi] Dhama, Kuldeep et al. “Biomarkers in Stress Related Diseases/Disorders: Diagnostic, Prognostic, and Therapeutic Values.” Frontiers in molecular biosciences vol. 6 91. 18 Oct. 2019, doi:10.3389/fmolb.2019.00091
[xxvii] Dhama, Kuldeep et al. “Biomarkers in Stress Related Diseases/Disorders: Diagnostic, Prognostic, and Therapeutic Values.” Frontiers in molecular biosciences vol. 6 91. 18 Oct. 2019, doi:10.3389/fmolb.2019.00091
[xxviii] Piazza, Jennifer R et al. “Frontiers in the use of biomarkers of health in research on stress and aging.” The journals of gerontology. Series B, Psychological sciences and social sciences vol. 65,5 (2010): 513-25. doi:10.1093/geronb/gbq049
[xxix] Ghiciuc, Cristina Mihaela et al. “Awakening responses and diurnal fluctuations of salivary cortisol, DHEA-S and α-amylase in healthy male subjects.” Neuro endocrinology letters vol. 32,4 (2011): 475-80.
[xxx] Slavish, Danica C et al. “Salivary markers of inflammation in response to acute stress.” Brain, behavior, and immunity vol. 44 (2015): 253-69. doi:10.1016/j.bbi.2014.08.008






