Non-alcoholic fatty liver disease (NAFLD), more currently identified as metabolic dysfunction-associated fatty liver disease (MAFLD) or metabolic dysfunction-associated steatotic liver disease (MASLD), affects at least 25% of the world’s population and is the most common chronic liver disease. Its main characteristics include (Guo 2022):
- Endoplasmic reticulum stress
- Hepatic steatosis in more than 5% of hepatocytes (not caused directly by alcohol or other liver damage factors)
- Lipid accumulation
- Lipotoxicity
- Metabolic risk factors
- e.g., obesity, metabolic syndrome, insulin resistance, type 2 diabetes, hyperlipidemia, hypertension
- Excess sugar and fat intake
- Mitochondrial damage and dysfunction
- Oxidative stress
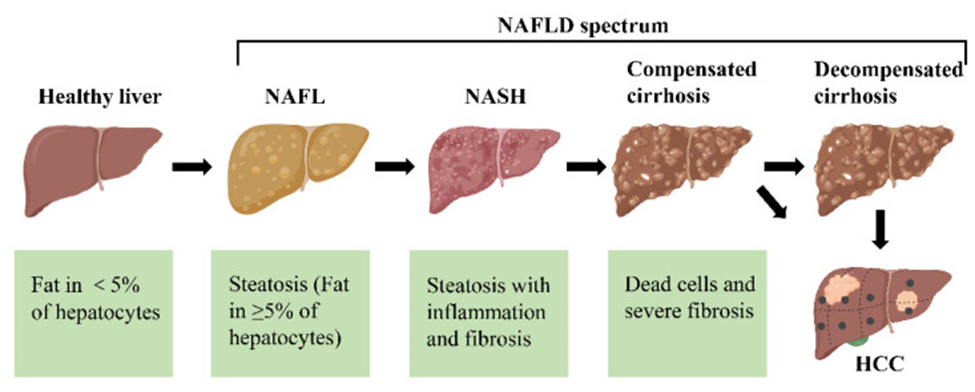
The different stages of non-alcoholic fatty liver disease (NAFLD). First, the healthy livers develop non-alcoholic fatty liver (NAFL) with hepatocellular steatosis as the main feature. If left untreated, NAFL may progress to a more severe form of non-alcoholic steatohepatitis (NASH), defined as inflammation and fibrosis in addition to hepatocellular steatosis. As the disease progresses, NASH may progress to cirrhosis and even to hepatocellular carcinoma (HCC).
Abnormal lipid accumulation in NAFLD/MAFLD/MASLD
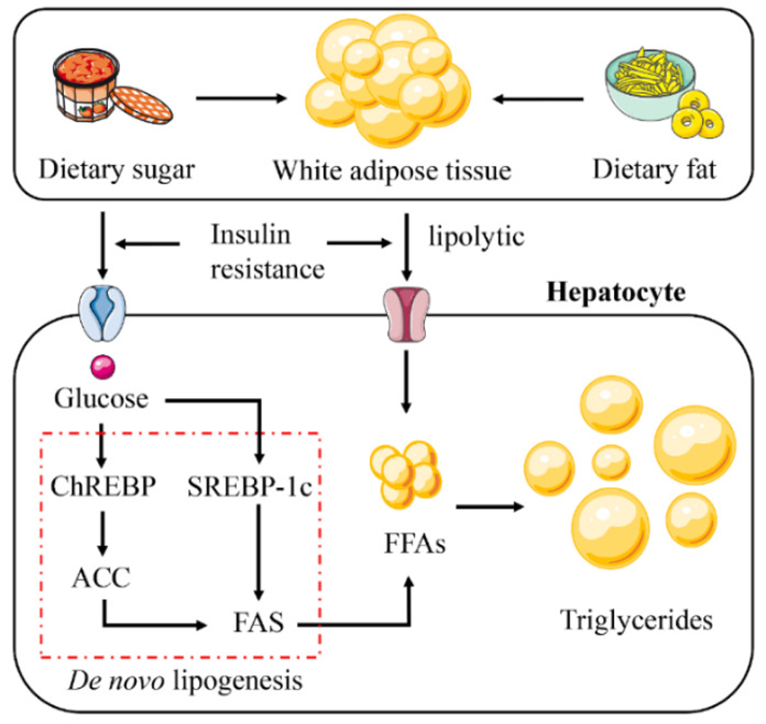
The increase in hepatic lipid accumulation is due to the absorption of large amounts of free fatty acids (FFAs) synthesized triglycerides by the liver from white adipose tissue (WAT), high-fat and high-sugar foods, and de novo lipogenesis (DNL). Insulin resistance plays a vital role in this process. Insulin resistance promotes glucose absorption and enhances the lipolysis of WAT. This leads to the activation of the DNL pathway. Abbreviations: ChREBP, carbohydrate response element binding protein; SREBP-1c, sterol regulatory element binding protein 1c; ACC, acetyl-CoA carboxylase; FAS, fatty acid synthase.
Source: Guo, Xiangyu et al. “Non-Alcoholic Fatty Liver Disease (NAFLD) Pathogenesis and Natural Products for Prevention and Treatment.” International journal of molecular sciences vol. 23,24 15489. 7 Dec. 2022, doi:10.3390/ijms232415489 This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
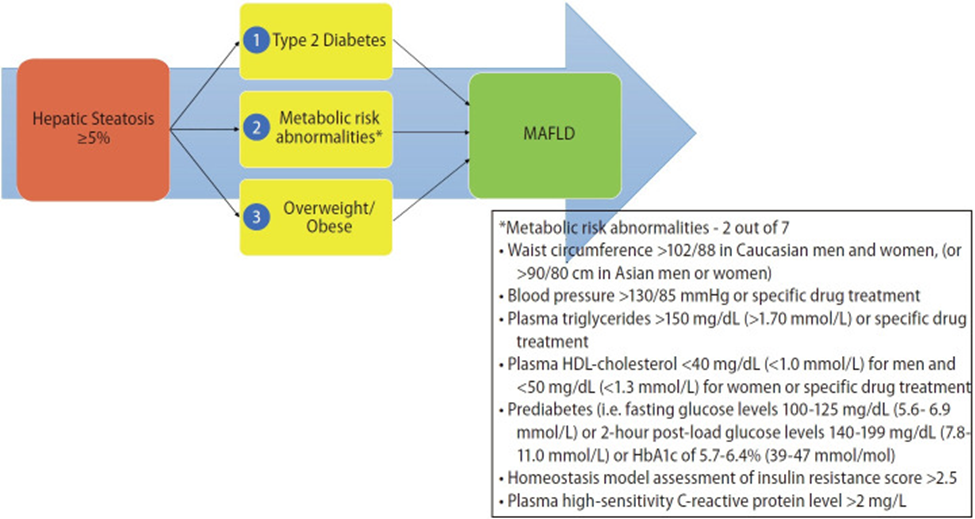
Criteria defining metabolic risk factors (Eslam J Hepatol. 2020):
A panel of international experts from 22 countries propose a new definition that is both comprehensive yet simple for the diagnosis of MAFLD and is independent of other liver diseases. The criteria are based on evidence of hepatic steatosis, in addition to one of the following three criteria, namely overweight/obesity, presence of type 2 diabetes mellitus, or evidence of metabolic dysregulation.
Increased cardiometabolic and MAFLD risk defined presence of at least two of the following metabolic at-risk criteria:
- Waist circumference ≥102/88 cm in Caucasian men and women or ≥90/80 cm in Asian men and women)*
- Blood pressure ≥ 130/85 mmHg or specific drug treatment.
- Plasma triglycerides ≥150 mg/dL (≥70 mmol/l) or specific drug treatment.
- Plasma HDL-cholesterol <40 mg/dL (<1.0 mmol/L) for men and <50 mg/dL (<1.3 mmol/L) for women or specific drug treatment.
- Prediabetes, i.e.,
- Fasting glucose levels 100 to 125 mg/dL (5.6 to 6.9 mmol/L)
- or 2-hour post-load glucose levels 140 to 199 mg/dL (7.8 to 11.0 mmol)
- or HbA1c 5.7% to 6.4% (39–47 mmol/mol)).
- Homeostasis model assessment (HOMA)-insulin resistance score ≥5.
- Plasma high-sensitivity C-reactive protein (hs-CRP) level >2 mg/L.
MAFLD may be present with other liver disorders:
- Any other cause for liver disease, e.g., alcohol-use disorder
- Defined as consumption of >3 drinks per day in men and >2 drinks per day in women, or binge drinking (defined as >5 drinks in males and >4 drinks in females, consumed over a 2-hour period)**, as defined by the National Institute of Alcoholism and Alcohol Abuse
- Viral infection (HIV, HBV and HCV)
- Autoimmune hepatitis
- Inherited liver disorders
- Drug-induced liver injury or other known liver disease
* The AHA/NHLBI guidelines for metabolic syndrome recognize an increased risk for cardiovascular disease and diabetes at waist-circumference thresholds of ≥94 cm in men and ≥80 cm in women and identify these as optional cut points for Caucasian individuals or populations with increased insulin resistance.
** These thresholds are derived from quantities beyond which a person is at more risk for alcohol related liver disease and may be in excess of the quantity needed to modify disease progression in MAFLD. This requires further study.
Source: Eslam, Mohammed et al. “A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement.” Journal of hepatology vol. 73,1 (2020): 202-209. doi:10.1016/j.jhep.2020.03.039. This manuscript is made available under the CC BY NC user license https://creativecommons.org/licenses/by-nc/4.0/
NAFLD criteria
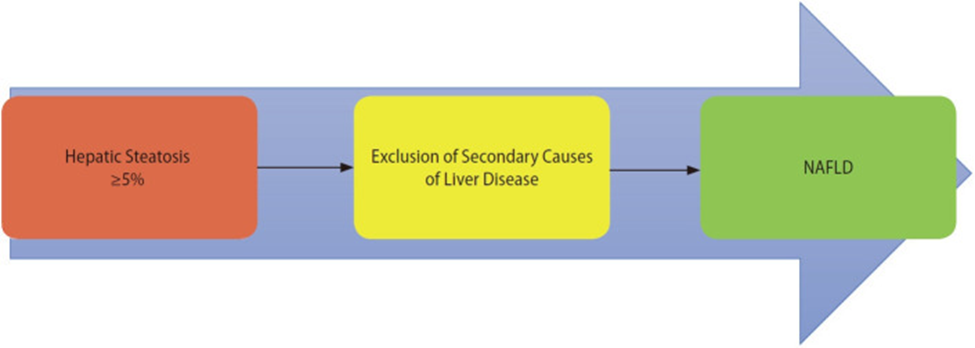
MAFLD criteria
Source: Gofton, Cameron et al. “MAFLD: How is it different from NAFLD?.” Clinical and molecular hepatology vol. 29,Suppl (2023): S17-S31. doi:10.3350/cmh.2022.0367 This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/)
Proposed transition from NAFLD to MAFLD terminology (Gofton 2023):
- The switch to a set of positive diagnostic criteria results in the ability to detect all underlying liver diseases, particularly in patients without apparently clear metabolic features.
- MAFLD diagnosis has been crucial in identifying higher-risk patients who would benefit from targeted management.
- The most significant difference between NAFLD and the diagnosis of MAFLD is the removal of the exclusion of concurrent liver disease to entertain the diagnosis.
- Multiple studies have shown the synergistic effects of comorbid liver disease, including viral hepatitis, and concurrent alcohol usage; however, the exclusion of these in the diagnosis of NAFLD underpins a cognitive dissonance between these disease processes, attempting to exclude their contribution to individualized patient outcomes.
- MAFLD is unrelated to the presence or absence of other causes of liver disease.
- Recent studies assessing NAFLD vs. MAFLD have identified that asymptomatic atherosclerotic cardiovascular disease has an independent association on multivariable logistic regression models with MAFLD but not with NAFLD diagnosis.
- A data review of 13,083 NHANES subjects found that those who met MAFLD diagnostic criteria had statistically significant increases in metabolic comorbidities, liver enzymes, and non-invasive liver fibrosis scores compared to the NAFLD group.
- Broadening diagnostic criteria allows clinicians to identify and treat all the liver diseases that might exist in a given patient in a holistic manner.
Additional considerations
A meta-analysis of 22 observational studies comprising 379,801 subjects found that MAFLD was significantly associated with male gender, higher BMI, hypertension, diabetes, hypertriglyceridemia, lower HDL, elevated transaminases, and higher fibrosis scores than NAFLD. In general, there was a greater chance of being diagnosed with MAFLD versus NAFLD. However, researchers suggest that the current definition of MAFLD only accounted for 81.59% of those diagnosed with NAFLD in this analysis and that some patients may be missed using the proposed MAFLD criteria (Lim 2023).
Metabolic dysfunction-associated steatotic liver disease (MASLD)
The American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of Liver Diseases (EASL) have recently adopted the term “metabolic dysfunction-associated steatotic liver disease (MASLD)” instead of MAFLD. However, diagnostic criteria are similar (AASLD, EASL).
MASLD diagnostic criteria
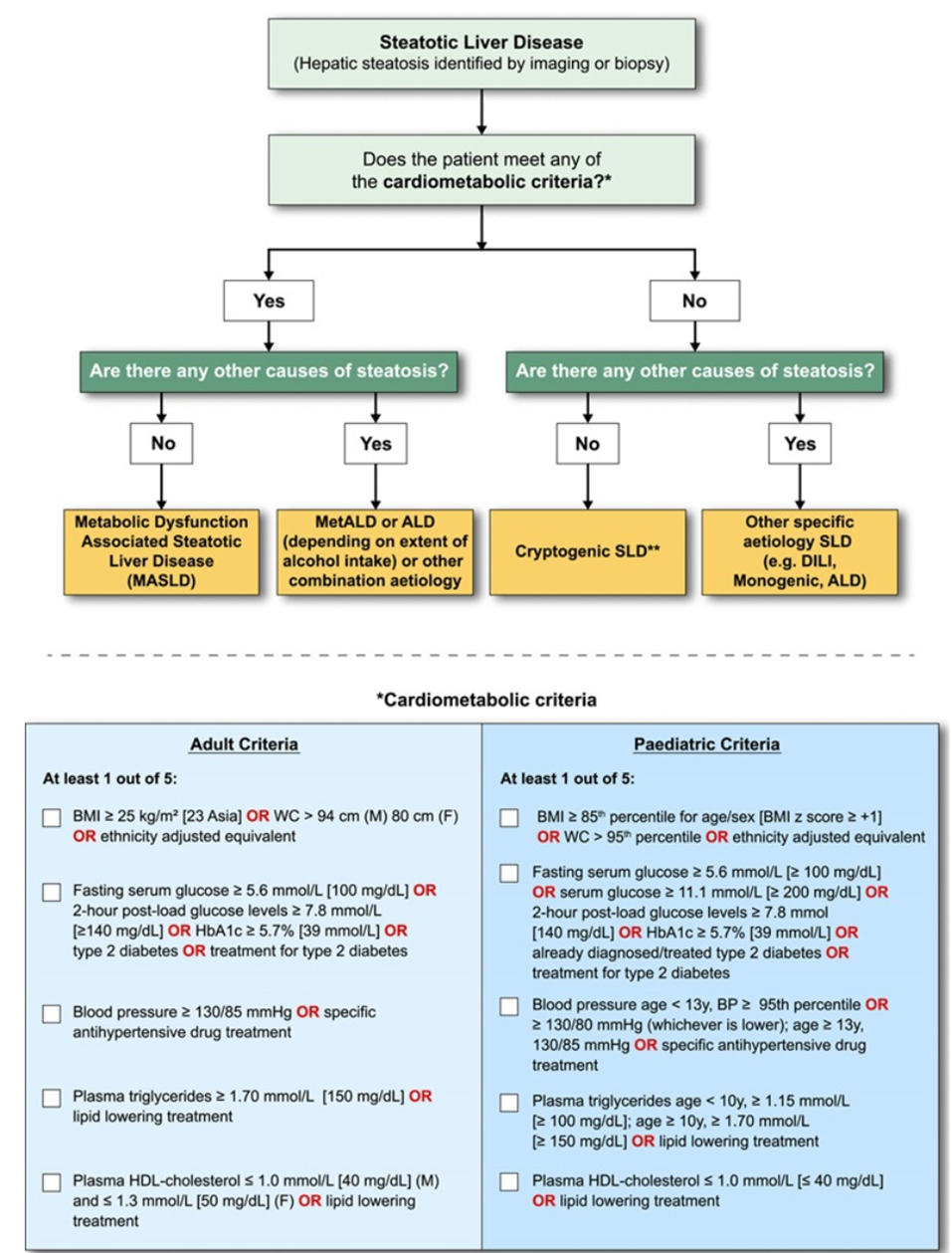
In the presence of hepatic steatosis, the finding of any CMRF would confer a diagnosis of MASLD if there are no other causes of hepatic steatosis. If additional drivers of steatosis are identified, then this is consistent with a combination etiology. In the case of alcohol, this is termed MetALD or ALD, depending on extent of alcohol intake. In the absence of overt cardiometabolic criteria, other aetiologies must be excluded, and if none is identified, this is termed cryptogenic SLD although, depending on clinical judgment, it could also be deemed to be possible MASLD and, thus, would benefit from periodic reassessment on a case-by-case basis. In the setting of advanced fibrosis/cirrhosis, steatosis may be absent, requiring clinical judgment based on CMRFs and absence of other aetiologies. Abbreviations: ALD, alcohol-associated/related liver disease; BMI, body mass index; BP, blood pressure; CMRF, cardiometabolic risk factors; DILI, drug-induced liver disease; MetALD, metabolic dysfunction and alcohol associated steatotic liver disease; SLD, steatotic liver disease; WC, waist circumference.
Source: Rinella ME, Lazarus JV, Ratziu V, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature [published online ahead of print, 2023 Jun 24]. Hepatology. 2023;10.1097/HEP.0000000000000520. doi:10.1097/HEP.0000000000000520 Under a Creative Commons license
The terms MAFLD and MASLD have more in common than not and have achieved the objective of a more appropriate name for the condition (Chan 2023)
|
MAFLD |
MASLD |
|
|
Positive diagnostic criteria (i.e., defines what the disease is rather than what it is not) |
Yes |
Yes |
|
Attributes the condition to its etiology |
Yes |
Yes |
|
Criteria |
Hepatic steatosis detected either by imaging techniques, blood biomarkers/scores, or liver history, plus |
Hepatic steatosis detected by imaging or biopsy, plus at least 1 of 5: |
|
Presence of other concomitant liver diseases |
Other concomitant liver diseases retain their own term |
Falls under a separate group (i.e., MetALD* or other combination etiology) |
*MetALD, i.e., weekly intake 140–350 g for females, 210–420 g for males (average daily 20–50 g for females, 30–60 g for males). MAFLD, metabolic dysfunction-associated fatty liver disease; MASLD, metabolic dysfunction-associated steatotic liver disease; HDL, high-density lipoprotein; HbA1c, glycosylated hemoglobin; HOMA-IR, homeostatic model for assessment of insulin resistance; hs-CRP, high sensitivity C-reactive protein; BMI, body mass index; MetALD, MASLD and increased alcohol intake.
Source: Chan, Wah-Kheong et al. “Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A State-of-the-Art Review.” Journal of obesity & metabolic syndrome vol. 32,3 (2023): 197-213. doi:10.7570/jomes23052. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0)
Lifestyle Factors
Metabolic dysfunction-associated fatty/steatotic liver disease can advance to a more severe, life-threatening form called metabolic dysfunction-associated steatohepatitis (MASH) or even hepatocellular carcinoma and should be identified and addressed early. Appropriate holistic interventions include (Chan 2023):
- Maintenance of a healthy weight and reduction of abdominal adiposity
- Promotion of regular exercise, increased physical activity, and sarcopenia prevention
- Optimization of nutrition intake, e.g., the Mediterranean Diet
- Minimization of cardiovascular risk factors, e.g., insulin resistance, metabolic syndrome, type 2 diabetes, inflammation, oxidative stress,, hypertension, and atherogenic dyslipidemia
- Smoking cessation
References
AALSD. New MASLD Nomenclature. https://www.aasld.org/new-masld-nomenclature
Chan, Wah-Kheong et al. “Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A State-of-the-Art Review.” Journal of obesity & metabolic syndrome vol. 32,3 (2023): 197-213. doi:10.7570/jomes23052.
Eslam, Mohammed et al. “A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement.” Journal of hepatology vol. 73,1 (2020): 202-209. doi:10.1016/j.jhep.2020.03.039
EASL. Multinational liver societies announce new “Fatty” liver disease nomenclature that is affirmative and non-stigmatising. https://easl.eu/news/new_fatty_liver_disease_nomenclature-2/
Gofton, Cameron et al. “MAFLD: How is it different from NAFLD?.” Clinical and molecular hepatology vol. 29,Suppl (2023): S17-S31. doi:10.3350/cmh.2022.0367. This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/)
Guo, Xiangyu et al. “Non-Alcoholic Fatty Liver Disease (NAFLD) Pathogenesis and Natural Products for Prevention and Treatment.” International journal of molecular sciences vol. 23,24 15489. 7 Dec. 2022, doi:10.3390/ijms232415489
Lim, Grace En Hui et al. “An Observational Data Meta-analysis on the Differences in Prevalence and Risk Factors Between MAFLD vs NAFLD.” Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association vol. 21,3 (2023): 619-629.e7. doi:10.1016/j.cgh.2021.11.038