Welcome to part 1 of the ODX B12 Deficiency Series. In this post, the ODX Research Team reviews the biochemistry and physiology of vitamin B12.
Vitamin B12 Biochemistry and Physiology
Dicken Weatherby, N.D. and Beth Ellen DiLuglio, MS, RDN, LDN
The ODX B12 Series
- Vitamin B12 Part 1 - Biochemistry and Physiology
- Vitamin B12 Part 2 - The Road to B12 Deficiency
- Vitamin B12 Part 3 - Biomarkers & Assessing B12 Deficiency
- Vitamin B12 Part 4 - How to Correct B12 Deficiency
Vitamin B12, a cobalt-containing compound known as cobalamin, is synthesized by bacteria yet is essential to humans.
The term cobalamin is a generic term for corrinoids (containing a corrin nucleus).[1]
This water-soluble vitamin (“vital amine”) occurs naturally as methylcobalamin (MeCbl), adenosylcobalamin (AdCbl), and hydroxocobalamin (OHCbl).
Cyanocobalamin, the synthetic form, contains a cyanide moiety that must be removed before being utilized in the body. Cyanocobalamin is especially contraindicated in smokers who already have overexposure to cyanide.[2]
Research indicates that all forms will be converted simply to cobalamin which in turn can be converted intracellularly to either methylcobalamin or adenosylcobalamin, the biologically active forms of B12. Interestingly, even if consumed as MeCbl or AdCbl, the methyl- and adenosyl- moieties will be removed and then MeCbl and AdCbl will be reassembled within the cell.[3]
Methylation of cobalamin depends on the availability of 5-methyltetrahydrofolate, an insufficiency of which can contribute to the insufficiency of methylcobalamin within the cell.[4]
Physiology and actions of B12 in the body
Body stores of vitamin B12 are maintained at approximately 2-3 mg. Enterohepatic circulation helps conserve the approximately 0.5-5 ug of B12 that is released in bile each day.[5] Therefore, an abrupt decrease in B12 intake should not cause an acute deficiency in most individuals.
When B12 is consumed in food, it must be “liberated” through adequate chewing and then processed at the gastric level. The low pH environment created by hydrochloric acid in the stomach enables gastric enzymes to cleave B12 from food sources.[6]
Dietary B12 is bound in the saliva and upper GI tract by the carrier protein haptocorrin and transferred to gastric-derived intrinsic factor which facilitates its absorption in the distal ileum. Haptocorrin comes into play again in the serum where it carries the majority of B12 along with B12 analogs. However, it is the carrier protein transcobalamin that facilitates the uptake of B12 into cells and its measurement is most specific for B12 deficiency.[7]
An estimated 20-25% of cobalamin in circulation is bound to transcobalamin. This complex, called holotranscobalamin (holoTC), is the active form available to cells and is considered the best marker for B12 deficiency.[8] Holotranscobalamin is found to correlate well with erythrocyte B12.[9]
Vitamin B12 fundamentally functions as a cofactor with profound effects on human metabolism.[10] [11]
- B12 functions as a cofactor for enzymes involved in many biochemical functions including macronutrient metabolism, myelination, and the processing of S-adenosylmethionine (SAM) and homocysteine.
- Without B12, homocysteine can accumulate increasing the risk of cardiovascular disease.
- Vitamin B12 is recharged by the methyltetrahydrofolate form of folate.
- B12 and folate support the formation of blood cells (hemopoiesis)
- B12 as MeCbl is involved in childhood brain development
- B12 deficiency leads to a buildup of methylmalonic acid and disruption of the metabolism of carbohydrates, fatty acids, amino acids, propionate, urea, and neuronal myelin.
- Elevated methylmalonic acid is more specific for B12 deficiency than elevated homocysteine as 2 other pathways are available for processing homocysteine.
- The corrin ring at the base of the cobalamin molecule is somewhat similar to the ring structure at the base of heme and of chlorophyll, two compounds vital to humans and plants respectively.
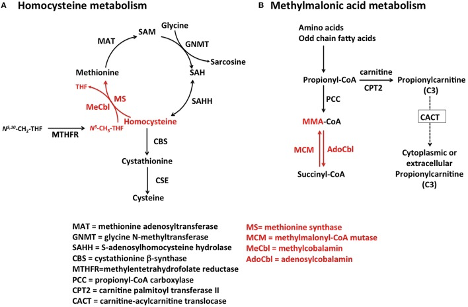
"Pathways for Hcy and MMA metabolism in humans. (A) Homocysteine is a branch-point metabolite at the intersection of either the remethylation or the transsulfuration pathways. Thus, Hcy homeostasis relies on three different biochemical reactions [MS, cystathionine β-synthase (CBS) and S-adenosylhomocysteine hydrolase (SAHH)], two of which (CBS and SAHH) are independent of vitamin B12. In addition to nutritional deficiency of vitamin B12, elevation of Hcy in plasma may arise from reduced function of CBS and MTHFR, as well as nutritional deficiencies of folate. (B) MMA is produced during catabolism of odd-chain fatty acids and amino acids in the mitochondrion. Propionyl-CoA is the precursor of MMA in a reaction catalyzed by propionyl-CoA carboxylase (PCC). Inborn errors of PCC lead to propionic acidemia. Likewise, mutations in AdoCbl-dependent MCM lead to a buildup of MMA-CoA and inhibition of PCC that manifests as increased propionyl-CoA and so of propionic acid the circulation. Propionylcarnitine can also be transported out of the cell to reach systemic circulation. Propionylcarnitine is a first-line test in newborn screening."
Source: Hannibal, Luciana et al. “Biomarkers and Algorithms for the Diagnosis of Vitamin B12 Deficiency.” Frontiers in molecular biosciences vol. 3 27. 27 Jun. 2016.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
Specific functions of the active intracellular forms of B12:[12] [13]
5΄-Deoxyadenosylcobalamin (adenosylcobalamin)
- Essential to Kreb’s cycle, metabolism of carbohydrates, fats, amino acids
- Serves as a cofactor for the mitochondrial enzyme, methylmalonyl CoA mutase.
- Methylmalonyl CoA mutase catalyzes the conversion of methylmalonyl CoA to succinyl CoA, an intermediate step in the conversion of propionate to succinate.
- This conversion is an important step in the oxidation of odd-chain fatty acids and in the catabolism of ketogenic amino acids.
- Essential to myelin synthesis and maintenance
5-Methylcobalamin (methylcobalamin)
- Essential for nucleic acid synthesis
- Cofactor for methionine synthase enzyme
- Facilitates conversion of homocysteine to methionine (remethylates homocysteine)
- Serves as a cofactor in folate-dependent conversion of homocysteine to methionine, catalyzed by the MeCbl-dependent enzyme methionine synthase.
- Methionine is required for incorporation into proteins and for synthesis of the universal methyl donor, SAM.
- The methionine synthase reaction also converts methyltetrahydrofolate to tetrahydrofolate:
- Tetrahydrofolate is subsequently converted to methylenetetrahydrofolate after condensation with formate or by a one-carbon transfer during conversion of serine to glycine.
- Methylenetetrahydrofolate can be reduced again to form methyltetrahydrofolate or can serve as the one-carbon source for the de novo synthesis of thymidylate from deoxyuridylate, required for DNA replication.
- Insufficiency of methylcobalamin leads to “folate trap” and functional folate deficiency
- When B12 is deficient, the conversion of homocysteine and methyltetrahydrofolate to methionine and tetrahydrofolate is inhibited. Folate is trapped as methyltetrahydrofolate and therefore cannot serve as a substrate for thymidine synthesis. [14]
- Thus, a functional folate deficiency is induced
- This ultimately disrupts protein synthesis, cell proliferation, DNA synthesis, and red cell development, leading to megaloblastic (macrocytic) anemia.
Preliminary research suggests a role for cobalamin as a cofactor in the metabolism of nitric oxide. Researchers propose that the intermediate glutathionyl-cobalamin is an active form of B12 that participates in the production and function of nitric oxide and in turn affects cell membrane protection, immune, and vascular health.[15]
As you can see, B12 plays a functional role in metabolism throughout the body… from blood, to nerves, to DNA. Sufficiency of this essential vitamin could be the deciding factor between disease and optimal health.
We’ll be talking more about the insufficiency of B12, biomarker and biochemistry clues, food sources, supplementation, and more so stay tuned.
Next Up: Vitamin B12 Part 2 - The Road to B12 Deficiency
References
[1] Allen, Lindsay H et al. “Biomarkers of Nutrition for Development (BOND): Vitamin B-12 Review.” The Journal of nutrition vol. 148,suppl_4 (2018): 1995S-2027S.
[2] Thakkar, K, and G Billa. “Treatment of vitamin B12 deficiency-methylcobalamine? Cyancobalamine? Hydroxocobalamin?-clearing the confusion.” European journal of clinical nutrition vol. 69,1 (2015): 1-2.
[3] Paul, Cristiana, and David M Brady. “Comparative Bioavailability and Utilization of Particular Forms of B12 Supplements With Potential to Mitigate B12-related Genetic Polymorphisms.” Integrative medicine (Encinitas, Calif.) vol. 16,1 (2017): 42-49.
[4] Harrington, Dominic J. “Laboratory assessment of vitamin B12 status.” Journal of clinical pathology vol. 70,2 (2017): 168-173.
[5] Allen, Lindsay H et al. “Biomarkers of Nutrition for Development (BOND): Vitamin B-12 Review.” The Journal of nutrition vol. 148,suppl_4 (2018): 1995S-2027S.
[6] Allen, Lindsay H et al. “Biomarkers of Nutrition for Development (BOND): Vitamin B-12 Review.” The Journal of nutrition vol. 148,suppl_4 (2018): 1995S-2027S.
[7] Smith, A David et al. “Vitamin B12.” Advances in food and nutrition research vol. 83 (2018): 215-279.
[8] Nexo, Ebba, and Elke Hoffmann-Lücke. “Holotranscobalamin, a marker of vitamin B-12 status: analytical aspects and clinical utility.” The American journal of clinical nutrition vol. 94,1 (2011): 359S-365S.
[9] Hannibal, Luciana et al. “Biomarkers and Algorithms for the Diagnosis of Vitamin B12 Deficiency.” Frontiers in molecular biosciences vol. 3 27. 27 Jun. 2016,
[10] Smith, A David et al. “Vitamin B12.” Advances in food and nutrition research vol. 83 (2018): 215-279.
[11] Thakkar, K, and G Billa. “Treatment of vitamin B12 deficiency-methylcobalamine? Cyancobalamine? Hydroxocobalamin?-clearing the confusion.” European journal of clinical nutrition vol. 69,1 (2015): 1-2.
[12] Allen, Lindsay H et al. “Biomarkers of Nutrition for Development (BOND): Vitamin B-12 Review.” The Journal of nutrition vol. 148,suppl_4 (2018): 1995S-2027S.
[13] Thakkar, K, and G Billa. “Treatment of vitamin B12 deficiency-methylcobalamine? Cyancobalamine? Hydroxocobalamin?-clearing the confusion.” European journal of clinical nutrition vol. 69,1 (2015): 1-2.
[14] Allen, Lindsay H et al. “Biomarkers of Nutrition for Development (BOND): Vitamin B-12 Review.” The Journal of nutrition vol. 148,suppl_4 (2018): 1995S-2027S.
[15] Paul, Cristiana, and David M Brady. “Comparative Bioavailability and Utilization of Particular Forms of B12 Supplements With Potential to Mitigate B12-related Genetic Polymorphisms.” Integrative medicine (Encinitas, Calif.) vol. 16,1 (2017): 42-49.






