COVID-19: Initial indications and conventional interventions
Dicken Weatherby, N.D. and Beth Ellen DiLuglio, MS, RDN, LDN
Initial symptoms of COVID-19 are not unique and may overlap with many other disorders.
The ODX COVID-19 Series
- COVID-19: The pandemic that has become endemic
- COVID-19: Overlapping risk factors and chronic disease
- Nutritional status COVID-19: A covert factor in disease susceptibility
- COVID-19: Blood chemistry biomarker patterns - Clues and patterns lurking just under the surface
- COVID-19: Blood chemistry biomarker patterns - Down the research rabbit hole
- COVID-19: Blood Biomarkers - Neutrophils
- COVID-19: Blood Biomarkers - Albumin
- COVID-19: BloodBiomarkers - Cytokines
- COVID-19: Blood Biomarkers - Interleukin-6
- COVID-19: Blood Biomarkers - Interleukin-10
- COVID-19: Blood Biomarkers - Vitamin C
- COVID-19: Blood Biomarkers - Vitamin D
- COVID-19: Blood Biomarkers - Zinc
- Biomarker characteristics and blood type - help sharpen the COVID-19 clinical picture
- COVID-19: Initial indications and conventional interventions
- COVID-19: Long-term risk reduction - Naturopathic, functional medicine, and nutrition-based approaches to prevention
- A healthy diet is primary prevention for COVID-19
- You should have a gut feeling about COVID-19
- Beyond dietary food patterns…plant-based compounds may mitigate COVID-19 risk
- Targeted nutrition support in the battle against COVID-19
- Targeted nutrition support in COVID-19: Armed with vitamin C
- Targeted nutrition support in COVID-19: In sync with zinc
- Targeted nutrition support in COVID-19: Micronutrients and phytonutrients are important players
- Optimal Takeaways for improving immunity and reducing susceptibility to COVID-19
- Optimal - The Podcast: Episode 8 -Blood Biomarkers and Risk Factors for COVID-19 and its Comorbidities
Clinical presentation for COVID-19 can include[i]
- Cough, especially dry cough
- Diarrhea
- Dysgeusia
- Fatigue
- Fever
- Headache
- Hyposmia/anosmia (loss of sense of smell)
- Nausea
A Cochrane review differentiated four categories of symptoms: respiratory, gastrointestinal, cardiovascular, and systemic. While four major possible red flag symptoms were identified early on (fever, myalgia/arthralgia, fatigue, and headache),[ii] we now know that there may be significant asymptomatic transmission, making control of community spread very difficult. The emergence of significant mutations, including variations in the spike protein, make this novel coronavirus an even greater threat.
Testing [iii] [iv]
- Reverse transcription–polymerase chain reaction (PCR) testing identifies genetic material from the virus itself
- Nasopharyngeal, oral, and saliva (saliva may be more sensitive than nasopharyngeal swab[v])
- Stool sample
- Blood IgM, IgG measures antibodies to coronavirus
Initially, strict social distancing, hand-washing, and face and eye coverings were the main defense against COVID-19. As of April 1, 2021, three vaccines have been granted emergency use status in the United States.[vi]
Allopathic treatment for COVID-19 [vii] [viii] [ix]
- Anti-inflammatories
- Anti-virals
- Mesenchymal stem cells
- Monoclonal antibodies
- Convalescent plasma
- Remdesivir: antiviral therapy shortened median recovery time from 15 to 10 days for COVID-19 patients with evidence of lower respiratory tract infection.[x]
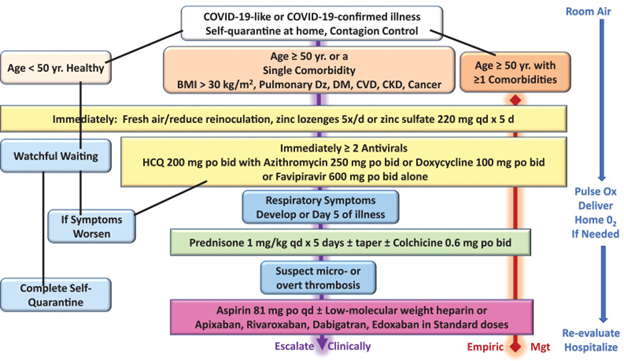
Source: McCullough, Peter A et al. “Pathophysiological Basis and Rationale for Early Outpatient Treatment of SARS-CoV-2 (COVID-19) Infection.” The American journal of medicine vol. 134,1 (2021): 16-22. doi:10.1016/j.amjmed.2020.07.003. Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID-19. The COVID-19 resource centre is hosted on Elsevier Connect, the company's public news and information website. Elsevier hereby grants permission to make all its COVID-19-related research that is available on the COVID-19 resource centre - including this research content - immediately available in PubMed Central and other publicly funded repositories, such as the WHO COVID database with rights for unrestricted research re-use and analyses in any form or by any means with acknowledgement of the original source. These permissions are granted for free by Elsevier for as long as the COVID-19 resource centre remains active
Conventional approaches to COVID-19 treatments may be life-saving in the acute setting. However, it is vitally important to incorporate fundamental nutrition and naturopathic approaches in the pre-, peri-, and post-COVID-19 period.
Research
[i] Gallo Marin, Benjamin et al. “Predictors of COVID-19 severity: A literature review.” Reviews in medical virology, e2146. 30 Jul. 2020, doi:10.1002/rmv.2146
[ii] Struyf, Thomas et al. “Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19 disease.” The Cochrane database of systematic reviews vol. 7,7 CD013665. 7 Jul. 2020, doi:10.1002/14651858.CD013665
[iii] Cao, Xuetao. “COVID-19: immunopathology and its implications for therapy.” Nature reviews. Immunology vol. 20,5 (2020): 269-270. doi:10.1038/s41577-020-0308-3
[iv] Ciotti, Marco et al. “The COVID-19 pandemic.” Critical reviews in clinical laboratory sciences vol. 57,6 (2020): 365-388. doi:10.1080/10408363.2020.1783198
[v] Ciotti, Marco et al. “The COVID-19 pandemic.” Critical reviews in clinical laboratory sciences vol. 57,6 (2020): 365-388. doi:10.1080/10408363.2020.1783198
[vi] CDC. Vaccines and immunizations.
[vii] Cao, Xuetao. “COVID-19: immunopathology and its implications for therapy.” Nature reviews. Immunology vol. 20,5 (2020): 269-270. doi:10.1038/s41577-020-0308-3
[viii] Joyner, Michael J et al. “Safety Update: COVID-19 Convalescent Plasma in 20,000 Hospitalized Patients.” Mayo Clinic proceedings vol. 95,9 (2020): 1888-1897. doi:10.1016/j.mayocp.2020.06.028
[ix] Liu, Miao et al. “Lessons learned from early compassionate use of convalescent plasma on critically ill patients with Covid-19.” Transfusion, 10.1111/trf.15975. 8 Aug. 2020, doi:10.1111/trf.15975
[x] Beigel, John H et al. “Remdesivir for the Treatment of Covid-19 - Final Report.” The New England journal of medicine vol. 383,19 (2020): 1813-1826. doi:10.1056/NEJMoa2007764






